Ultraspec R&D
We bridge academia and industries with our cutting-edge research
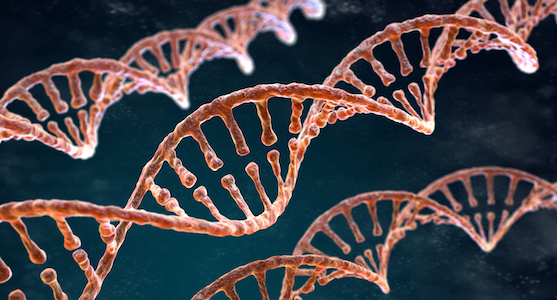
DNA methylation for early disease intervention
DNA methylation is a biological process that involves the addition of methyl groups to nucleobases on specific regions of a DNA sequence. Recent studies showed that DNA methylation age is highly related to chronological age. Capturing the methylation infomation could help us investigate possible health problems at the early stage and possibly pre-diagnose certain diseases. Our goal is to develop a new measure to predict an individual’s “biological” age based on their epigenetic clock, which indicates early onset of age-related diseases. The biological age predictor can be incorporated by clinicians to a standard annual checkup and used in combination with other health measures to determine an individual’s overall health and to enable early disease intervention.
Flux analysis of the Whole-body metabolic network
Traditionally researchers analyze metabolic systems using a simplified approximation of enzyme kinetics. as the result, the metabolic system could be represented by a series of non-linear kinetics equations (see for examples). However, it is not practical to find the kinetics equation for every single reaction in the system, especially when we simulate a large complicated system, for example, human metabolic network.
The alternative solution is which employs stoichiometric models of metabolism and mass spectrometry method to understand cellular physiology within particular boundaries and predict its metabolic capability over time. In our project we extended the method to understand the full scale of human metabolic network, including the major organs esential for human metabolism. Moreover, we applied morden AI framework to boost the simulation, which enable us to evaluate the impact of daily diet/vitamin intake, gendar difference, individual condition, etc. This method has great potential to help analysis and pre-experiment of drug effect in R & D stage.
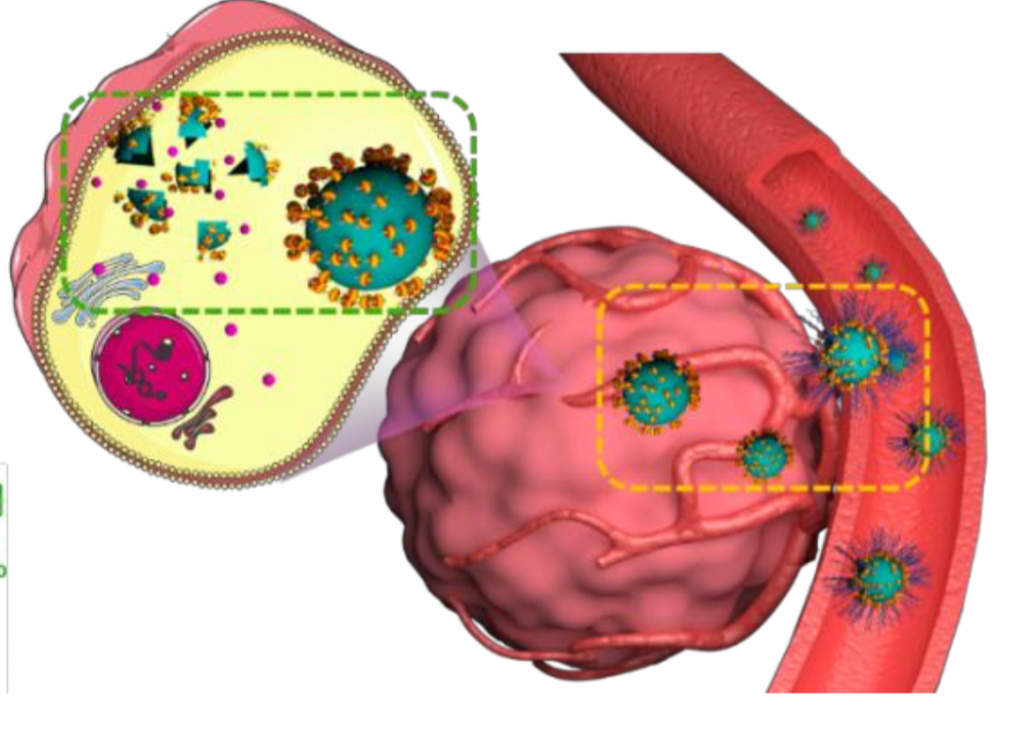
Supramolecular Nanomedicine for Selective Cancer Therapy
in vitro and in vivo
